Avery Biomedical Devices
Based on his pioneering research in cardiac pacemakers, William W.L. Glenn and his colleagues at the Yale University School of Medicine set out to create the first practical application of phrenic nerve pacing. Although first mentioned in 19664, the seminal work on the topic was an article titled “Radio-Frequency Electrophrenic Respiration. Long-term application to a patient with primary hypoventilation” which appeared in the March 1968 issue of the Journal of the American Medical Association5.
In collaboration with Roger E. Avery, Glenn’s prototypes were brought into commercial distribution by Avery Laboratories, Inc. in 1971. The Medical Device Amendment of 1976 mandated the classification and regulation of medical devices and in 1986, under FDA regulations the Medical Device Amendments were enacted into law. In January of 1987 Avery became one of the first to obtain PMA approval under this regulation.
Furthermore, in 1995 Avery breathing pacemaker system was one of the first products to obtain premarket approval as a Class III device under the requirements of the Active Implantable Medical Device Directive (90/385/EEC) for distribution within the European Union.
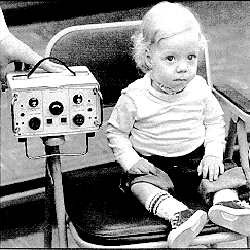
The Avery Model S-242 was the first commercially distributed diaphragm pacemaker.
