Recommended Physician Referrals
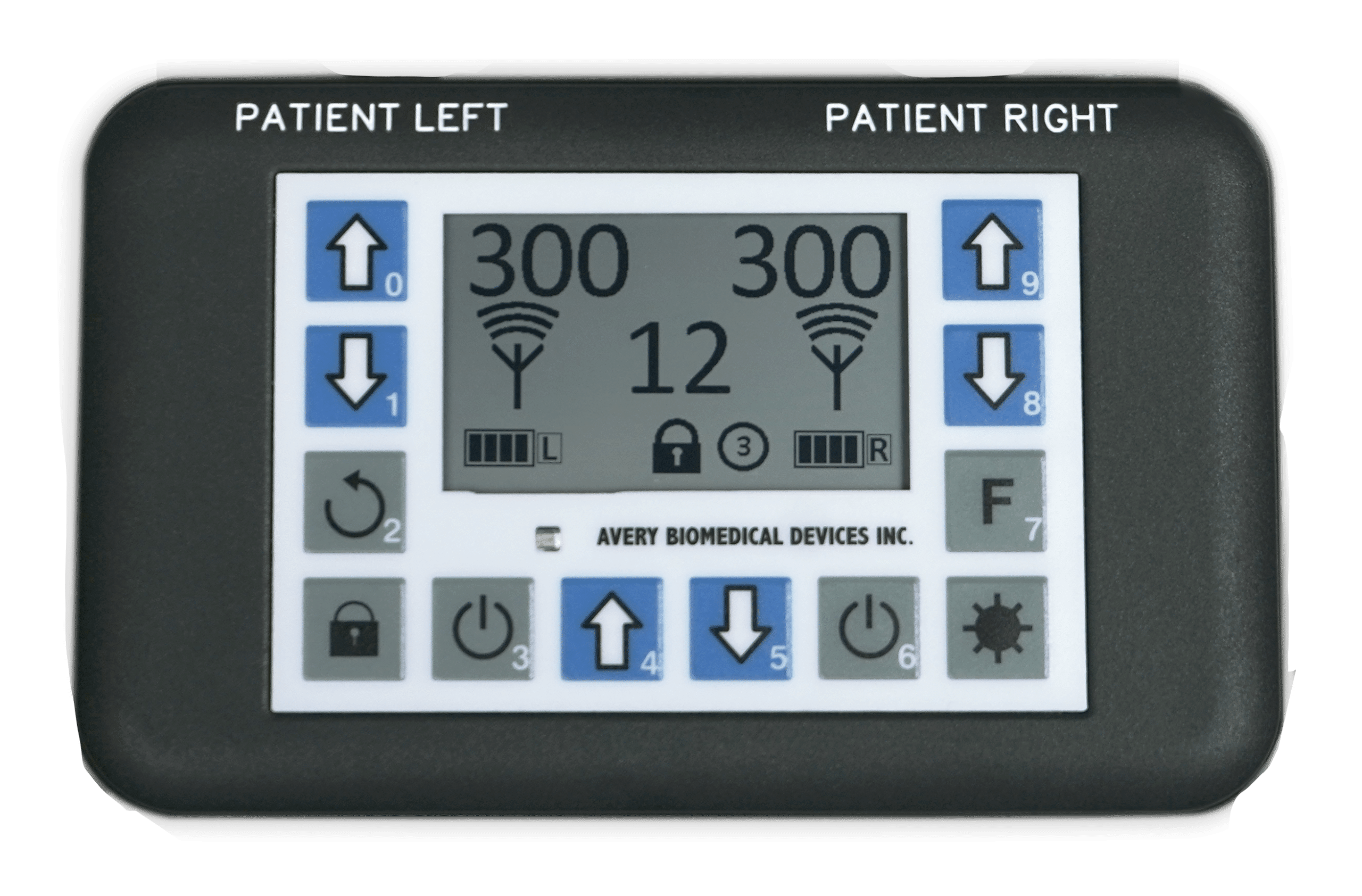
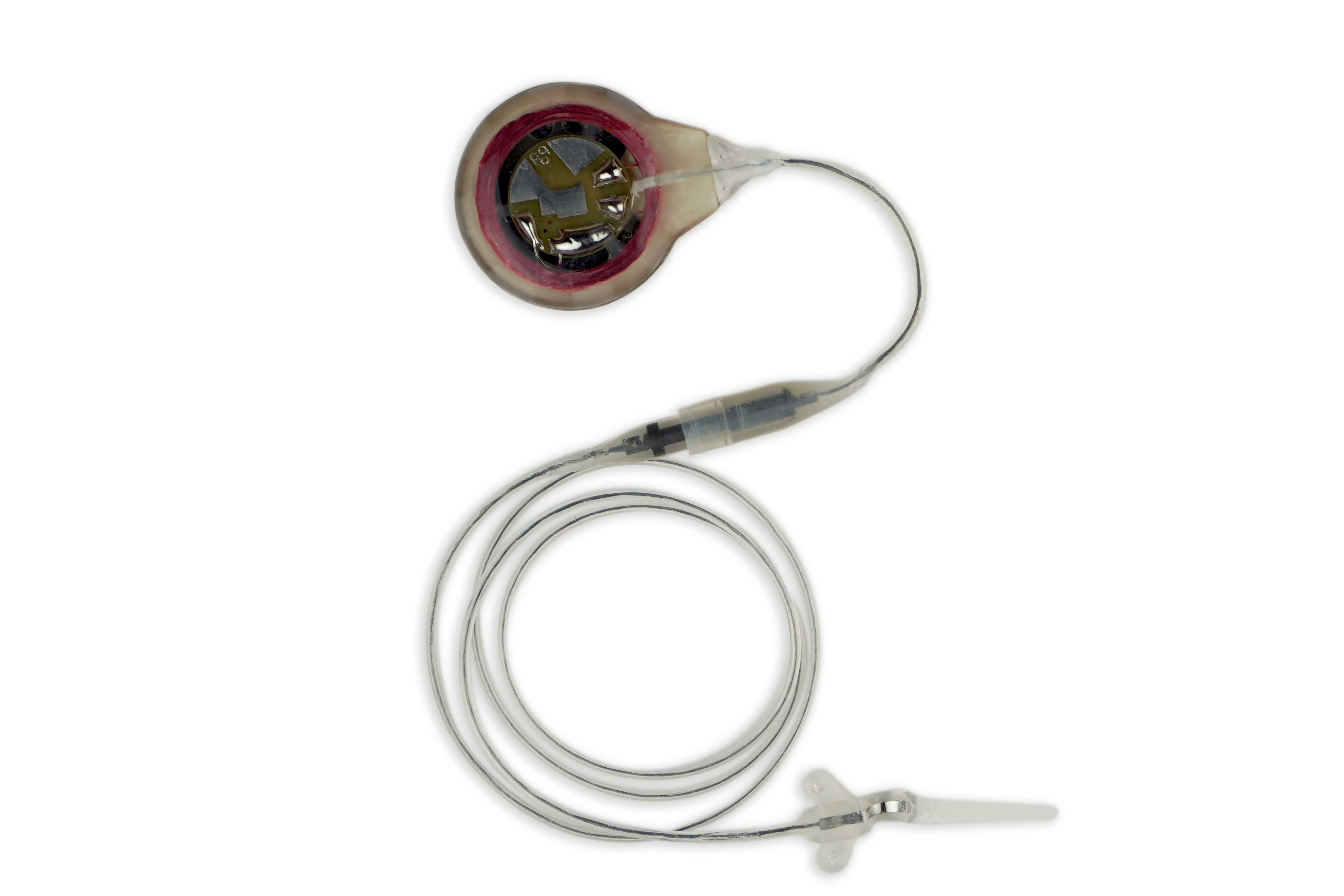
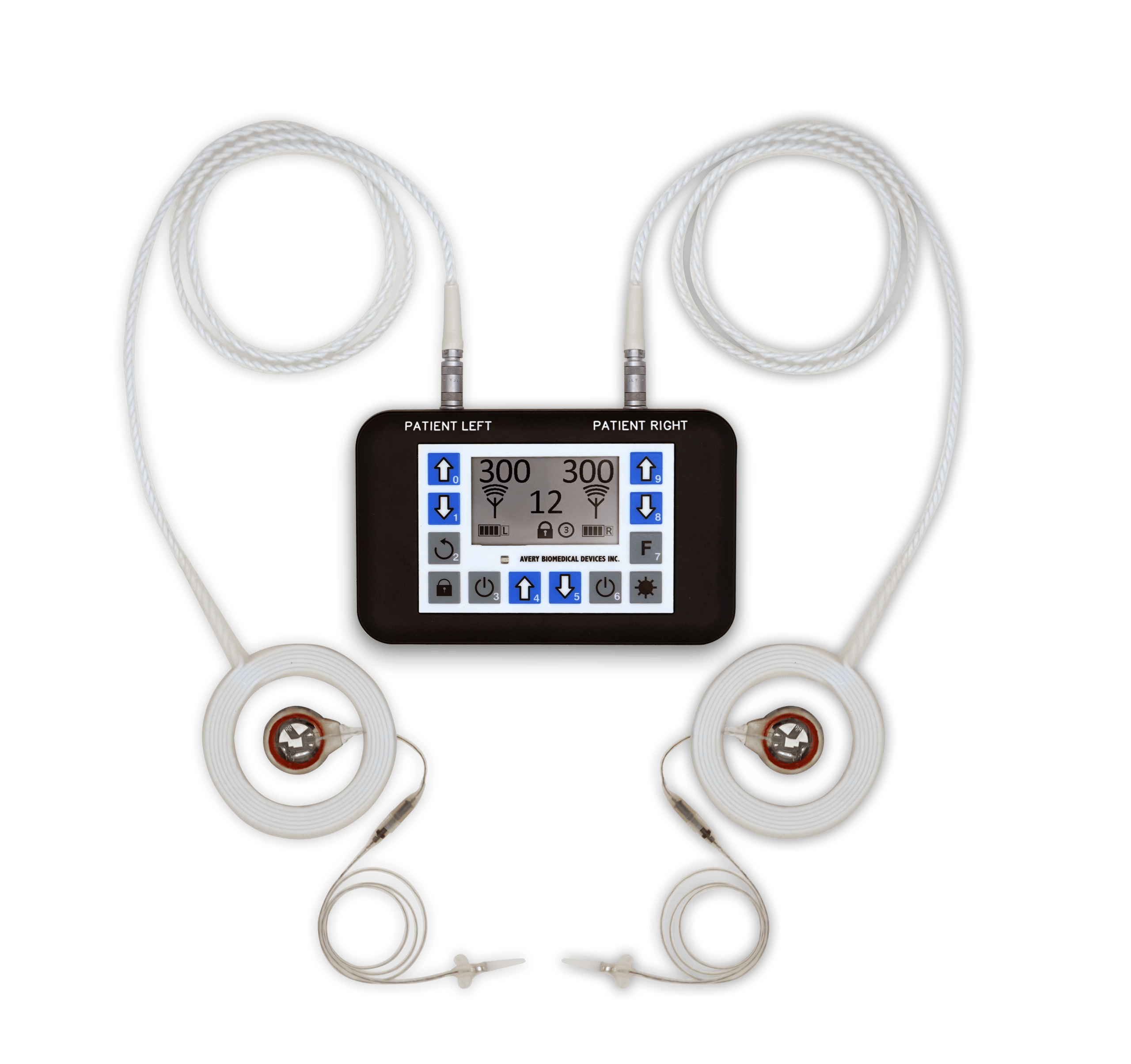
Avery Biomedical Devices works with physicians, specialists and a select number of surgeons throughout the United States for the implementation of the Avery Diaphragm Pacemaker. To find our top recommended specialists near you, please Contact Us directly through one of the following methods and we will be happy to connect you to a physician, specialist or surgeon that is familiar with the device and may further assist you and your specific needs.
The Avery Diaphragm Pacemaker has been implanted in patients in over 44 countries around the world.
For International Physicians please Contact Us to provide a referral.